Stars and Planets

—Atmospheres—
Chris Ormel

Roadmap module 6
Transitions
The change of an atom or molecule to a different energy state, which can result in the formation of a spectral line
(atomic) Hydrogen spectrum
Lyman, Balmer, Paschen, ... series
Stellar classification
characterize stars by their spectrum. O, B, A, F, G, K, M
(collisional) Ionization and excitation
Boltmann (excitation) and Saha (inonization) equations together determine the level populations of energy states
(exo)Planet atmospheres
Transparancy (size) atmosphere varies at different wavelengths
- electronic transitions
- vibrational transitions
- molecular transitions
- transmission spectrum
- greenhouse effect
Topics
- electronic/vibrational/molecular
- Saha equation
- Planet atmospheres
Transitions
read CO Ch. 8.1
-
Electronic.
Following Bohr's classical atomic model, energy levels for the electron are discretized as
![]()
where
![]() with
with
![]() the
Bohr radius.
This correspond to energy levels
the
Bohr radius.
This correspond to energy levels
![]() with transitions occurring at near-UV/visible wavelengths.
with transitions occurring at near-UV/visible wavelengths.
-
Vibrational.
Energy levels are
![]()
where v is the vibrational quantum number and the frequency is related to the bond force constant k (Hooke's law)
![]() . Energy levels a factor
. Energy levels a factor
![]() lower than electronic.
Lines appear at near-IR wavelengths
lower than electronic.
Lines appear at near-IR wavelengths
-
Rotational.
Energy levels are
![]()
(valid for a rigid rotating diatomic or linear molecule), where I is the moment of inertial and J is the rotational quantum number. Energy levels are lower by
![]() compared to electronic.
Transitions occur at (sub)millimeter wavelengths
compared to electronic.
Transitions occur at (sub)millimeter wavelengths
Transitions
read CO Ch. 8.1
-
Electronic.
Following Bohr's classical atomic model, energy levels for the electron are discretized as
![]()
where
![]() with
with
![]() the
Bohr radius.
This correspond to energy levels
the
Bohr radius.
This correspond to energy levels
![]() with transitions occurring at near-UV/visible wavelengths.
with transitions occurring at near-UV/visible wavelengths.
-
Vibrational.
Energy levels are
![]()
where v is the vibrational quantum number and the frequency is related to the bond force constant k (Hooke's law)
![]() . Energy levels a factor
. Energy levels a factor
![]() lower than electronic.
Lines appear at near-IR wavelengths
lower than electronic.
Lines appear at near-IR wavelengths
-
Rotational.
Energy levels are
![]()
(valid for a rigid rotating diatomic or linear molecule), where I is the moment of inertial and J is the rotational quantum number. Energy levels are lower by
![]() compared to electronic.
Transitions occur at (sub)millimeter wavelengths
compared to electronic.
Transitions occur at (sub)millimeter wavelengths

Transitions
read CO Ch. 8.1
-
Electronic.
Following Bohr's classical atomic model, energy levels for the electron are discretized as
![]()
where
![]() with
with
![]() the
Bohr radius.
This correspond to energy levels
the
Bohr radius.
This correspond to energy levels
![]() with transitions occurring at near-UV/visible wavelengths.
with transitions occurring at near-UV/visible wavelengths.
-
Vibrational.
Energy levels are
![]()
where v is the vibrational quantum number and the frequency is related to the bond force constant k (Hooke's law)
![]() . Energy levels a factor
. Energy levels a factor
![]() lower than electronic.
Lines appear at near-IR wavelengths
lower than electronic.
Lines appear at near-IR wavelengths
-
Rotational.
Energy levels are
![]()
(valid for a rigid rotating diatomic or linear molecule), where I is the moment of inertial and J is the rotational quantum number. Energy levels are lower by
![]() compared to electronic.
Transitions occur at (sub)millimeter wavelengths
compared to electronic.
Transitions occur at (sub)millimeter wavelengths

Transitions
The transition strength is determined by:
- the extent the upper level is populated; and
- the rate at which the excited state will decay by photon emission (Einstein-A)
(for an emission line).
-
Selection rules.
Not all transitions are allowed.
Dipole transitions require (i) a permanent dipole moment; and (ii)
![]() .
Symmetric molecules — notably H2 —
do not have a permanent dipole moment!
They can emit only through much weaker quadrapule transitions
.
Symmetric molecules — notably H2 —
do not have a permanent dipole moment!
They can emit only through much weaker quadrapule transitions
![]() .
In cold disk, the gas is molecular but H2 is essentially invisible
.
In cold disk, the gas is molecular but H2 is essentially invisible
Question:
How then do astronomers measure the mass of cold, molecular gas?
Transitions
The transition strength is determined by:
- the extent the upper level is populated; and
- the rate at which the excited state will decay by photon emission (Einstein-A)
(for an emission line).
-
Selection rules.
Not all transitions are allowed.
Dipole transitions require (i) a permanent dipole moment; and (ii)
![]() .
Symmetric molecules — notably H2 —
do not have a permanent dipole moment!
They can emit only through much weaker quadrapule transitions
.
Symmetric molecules — notably H2 —
do not have a permanent dipole moment!
They can emit only through much weaker quadrapule transitions
![]() .
In cold disk, the gas is molecular but H2 is essentially invisible
.
In cold disk, the gas is molecular but H2 is essentially invisible
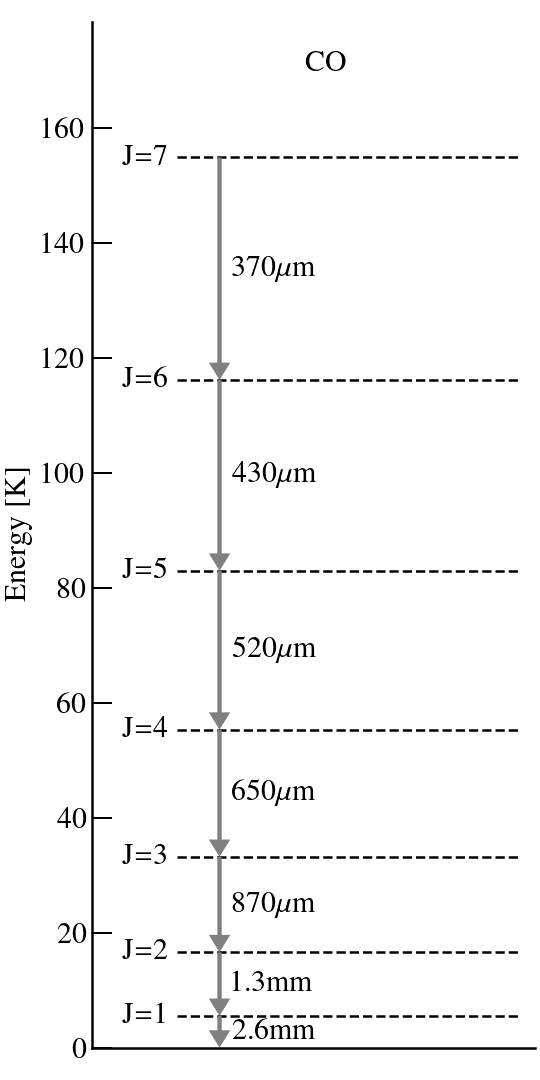
Figure on the right shows the energy levels of the CO molecule. At high temperatures — e.g., room temperatures — all levels will be occupied. But at lower T — e.g., ~10 K for molecular clouds or the interior regions of protoplanetary disks — only the lowest J levels will be.
Given the level populations and the transition rates — the Einstein-A coefficient — the line strength can be calculated.
Electronic transitions/H-atom
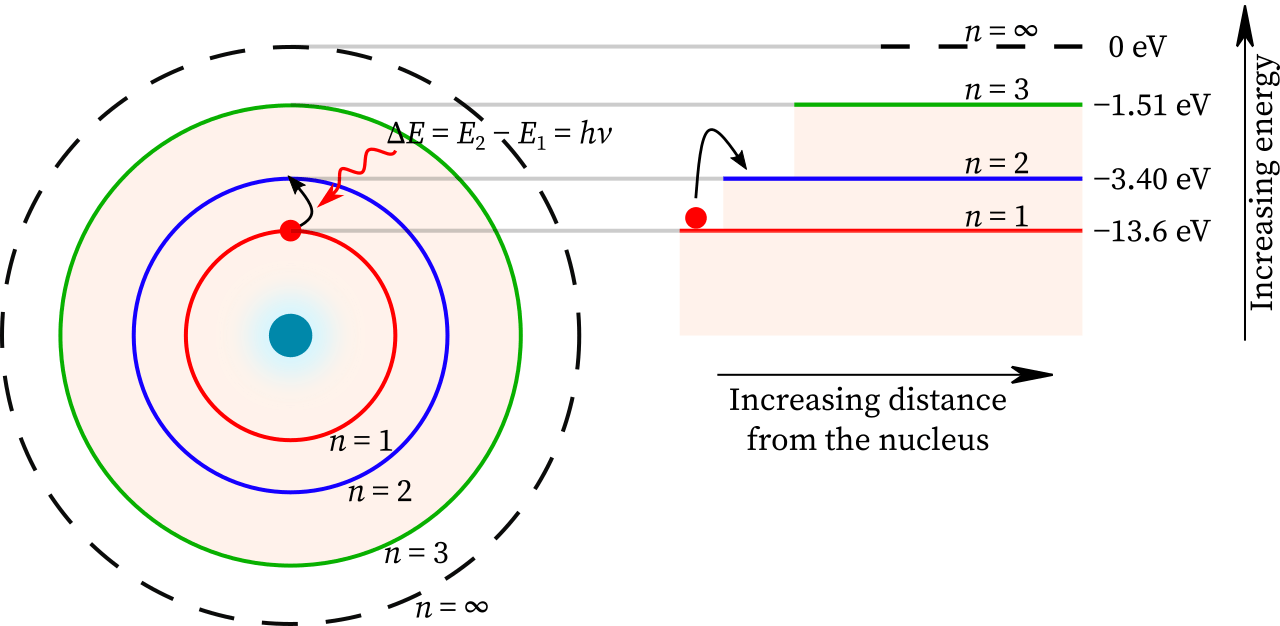
- (semi-classical) Bohr atomic model
Bohr postulated that angular momentum of the electron is quantized in units of
![]() .
The corresponding energy levels are
.
The corresponding energy levels are
![]()
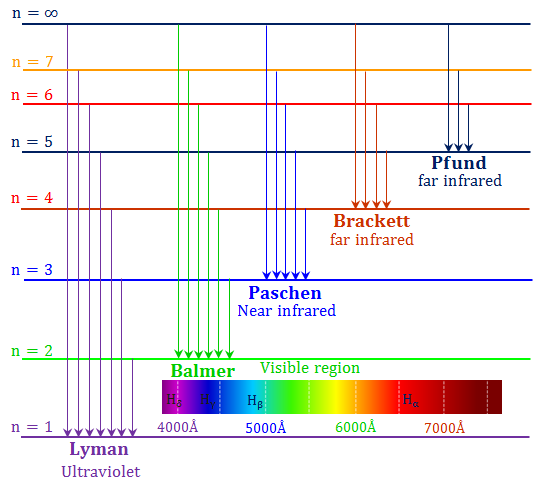
- For the hydrogen atom, the resulting transitions give rise to lines in the UV (n=1;
Lyman),
Visible (n=2;
Balmer)
and IR
Lines are further identified by a greek subscripts, e.g.
![]() for the
for the
![]() transition,
transition,
![]() for the
for the
![]() etc. The Balmer series appears in the visible.
etc. The Balmer series appears in the visible.
- In stellar atmospheres, these transitions appear in absorption
| name | abbr. | n | range λ [μm] |
|---|---|---|---|
| Lyman | Ly | 1 | 0.0912—0.122 |
| Balmer | Ba or H | 2 | 0.0365—0.656 |
| Paschen | Pa | 3 | 0.821—1.88 |
| Brackett | Br | 4 | 1.26—4.05 |
| Pfund | Pf | 5 | 2.28—7.46 |
| Humphreys | Hu | 6 | 3.28—12.4 |
Electronic transitions/H-atom
- (semi-classical) Bohr atomic model
Bohr postulated that angular momentum of the electron is quantized in units of
![]() .
The corresponding energy levels are
.
The corresponding energy levels are
![]()
- For the hydrogen atom, the resulting transitions give rise to lines in the UV (n=1;
Lyman),
Visible (n=2;
Balmer)
and IR
Lines are further identified by a greek subscripts, e.g.
![]() for the
for the
![]() transition,
transition,
![]() for the
for the
![]() etc. The Balmer series appears in the visible.
etc. The Balmer series appears in the visible.
- In stellar atmospheres, these transitions appear in absorption
Question:
Why in absorption?
ionization
read KW. Ch 14.1—2
The Boltzmann equation determines the occupation levels within an ionization state

where Ei denotes the energy level with respect to the ground state (E1=0), gi the statistical weight, and Z the partition function:

ionization
read KW. Ch 14.1—2
The Boltzmann equation determines the occupation levels within an ionization state
The Saha equation determines the occupation levels among ionization states:

where Eion is the ionization energy, and the Zint are internal partition functions.
ionization
read KW. Ch 14.1—2
The Boltzmann equation determines the occupation levels within an ionization state
The Saha equation determines the occupation levels among ionization states:

Applied to the ionization of hydrogen, we obtain

where AB=HI, A=HII, B=e,
 ,
,
 ,
,
 ,
,
 .
.
 is known as the
ionization fraction.
is known as the
ionization fraction.
Question: Do we see Balmer lines in the Sun's atmosphere?
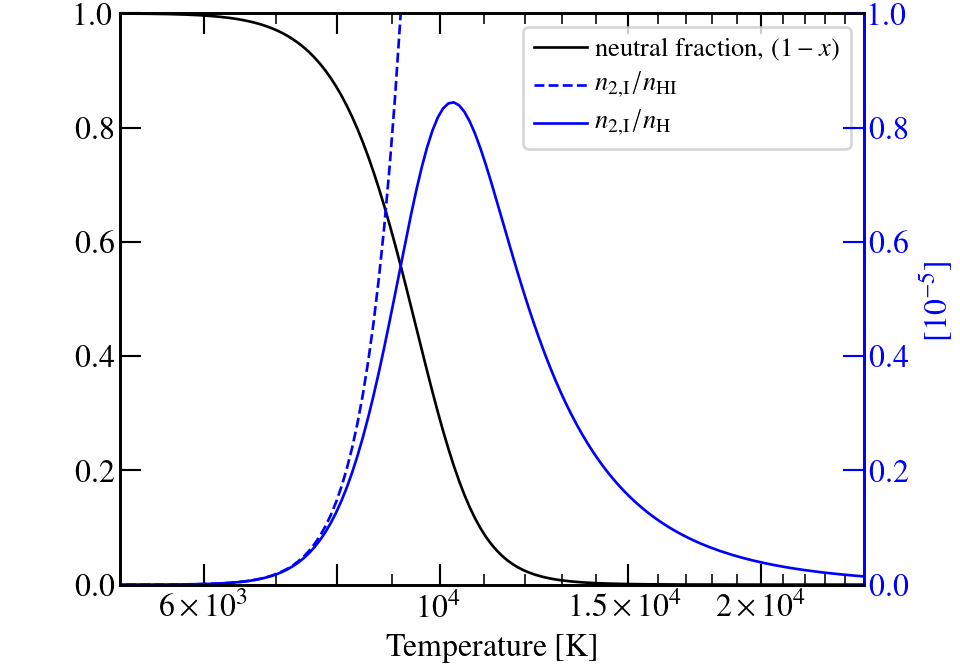
 was used for all T.
was used for all T.
ionization
read KW. Ch 14.1—2
The Boltzmann equation determines the occupation levels within an ionization state
The Saha equation determines the occupation levels among ionization states:

Applied to the ionization of hydrogen, we obtain

Note that in Astronomy roman numericals indicate the ionization stage:
- (single roman) for neutral (e.g., HI is neutral hydrogen)
- (roman numeral 2) for singly ionized (e.g., HII is ionized hydrogen)
- (roman 3) for double-ionized (e.g., OIII is O2+)
- etc.

 was used for all T.
was used for all T.
ionization
read KW. Ch 14.1—2
The Boltzmann equation determines the occupation levels within an ionization state
The Saha equation determines the occupation levels among ionization states:

Applied to the ionization of hydrogen, we obtain

Pressure ionization becomes important at high densities
This occurs when
 with a0 the Bohr radius. Or
with a0 the Bohr radius. Or
 Above these densities, everything is ionized.
Above these densities, everything is ionized.

 was used for all T.
was used for all T.
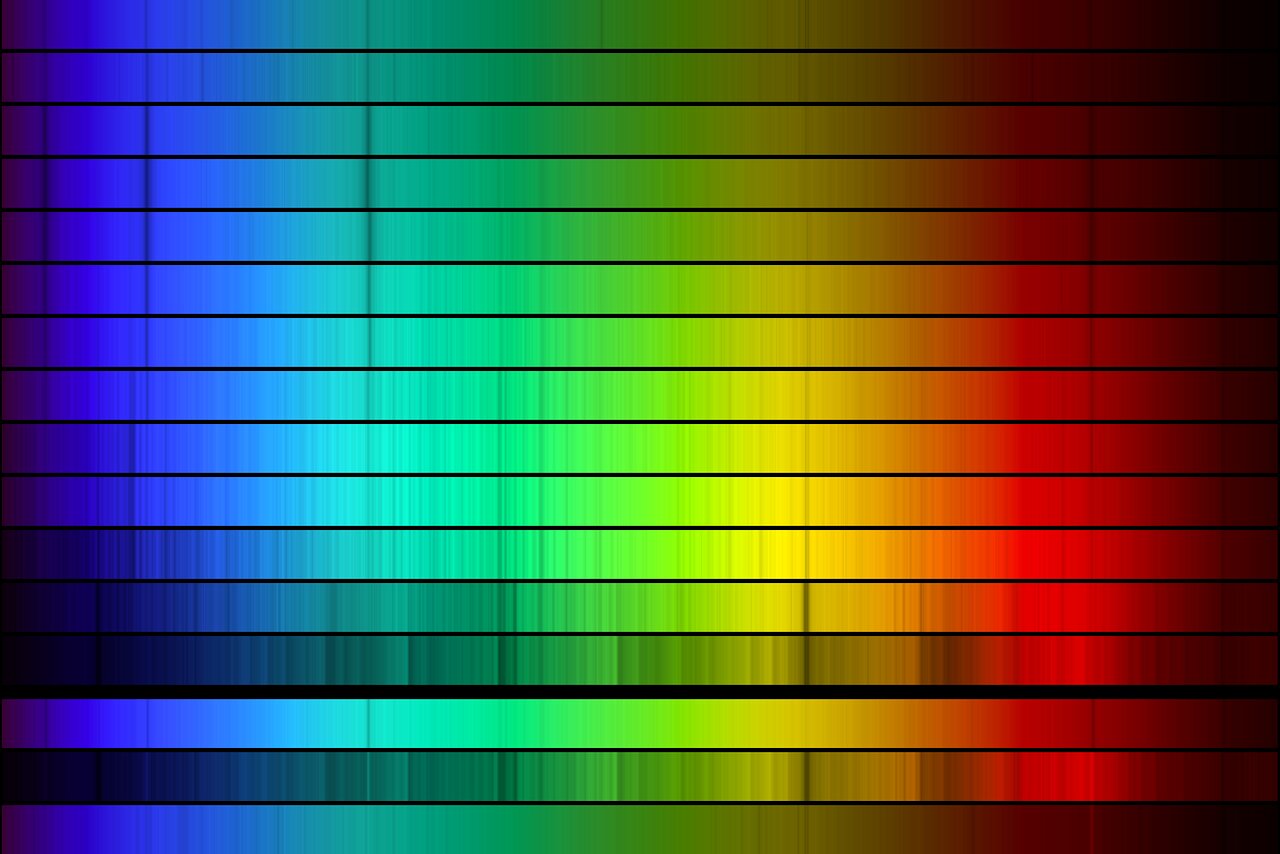
stellar classification
read CO Ch. 8

| Type | T(K) | Spectral lines |
|---|---|---|
| O | >25,000 | Neutral and ionized He |
| B | 11,000—25,000 | Neutral He, some H |
| A | 7,500—11,000 | strong H; ionized metal (Ca II, Mg II) |
| F | 6,000—7,500 | weak H; ionized metal |
| G | 5,000—6,000 | ionized and neutral metal |
| K | 3,500—6,000 | strong metal |
| M | <3,500 | molecules (TiO, MgH) |
| Stellar classes by spectral line prominence. See Table 8.1 of CO | ||
planet atmospheres
Earth's atmosphere consist of several layers
- exosphere. Here the particle mean free path exceeds the pressure scaleheight
![]()
- thermosphere. Very rarified layer. The ISS and the Tiangong space station (天宫) occupy this layer at ~400 km
- mesosphere. The energy balance is such that the temperature decreases again with height.
- stratosphere. Contains the ozone (O3) layer. Temperature rises with height due to UV absorption (and limited cooling). Convectively stable.
- troposphere. The lowest layer most of its mass, clouds, weather, etc. Temperature decreases with height (heated from below).

atmospheres
— read CO 19.3 —
The equilibrium temperature of a body at distance d from the star is

where a is the
albedo
— the amount of stellar light that is reflected.
For the Earth,
 .
However, this expression underestimate the surface temperature of the Earth, due to the greenhouse effect.
.
However, this expression underestimate the surface temperature of the Earth, due to the greenhouse effect.
With the
greenhouse effect
, the surface temperature increases

where τIR is the opacity in the infrared
Note. — this is essentially the Eddington approximation

atmospheres
— read CO 19.3 —
The equilibrium temperature of a body at distance d from the star is

where a is the
albedo
— the amount of stellar light that is reflected.
For the Earth,
 .
However, this expression underestimate the surface temperature of the Earth, due to the greenhouse effect.
.
However, this expression underestimate the surface temperature of the Earth, due to the greenhouse effect.
With the
greenhouse effect
, the surface temperature increases

where τIR is the opacity in the infrared
Note. — this is essentially the Eddington approximation

greenhouse effect
Outgoing thermal IR radiation trapped by vibrational transitions from molecules as CO2 and H2O!
Although the heat trapping principle is solid (it should not be disputed!), complexity arises in understanding feedback effects
- enhanced concentration of H2O (+)
- ice melting (+)
- arctic methane release (thawing permafrost) (+)
- carbon cycle (?)
- clouds (?)
low clouds contribute to albedo; high clouds trap IR-radiation
greenhouse effect
Outgoing thermal IR radiation trapped by vibrational transitions from molecules as CO2 and H2O!
Although the heat trapping principle is solid (it should not be disputed!), complexity arises in understanding feedback effects
- enhanced concentration of H2O (+)
- ice melting (+)
- arctic methane release (thawing permafrost) (+)
- carbon cycle (?)
- clouds (?)
low clouds contribute to albedo; high clouds trap IR-radiation

atmospheric escape
molecules escape at the
exobase,
 when their velocity exceeds the escape velocity (Jeans escape).
This results in a mass flux of
when their velocity exceeds the escape velocity (Jeans escape).
This results in a mass flux of

where
 is the ratio between the escape velocity and the thermal velocity
is the ratio between the escape velocity and the thermal velocity
exoplanet atmospheres
- Three types of spectra:
- emission spectrum
the spectrum arising from the brightness of the object itself
- transmission spectra
the spectrum arising when radiation of a (brighter) background object passes through a foreground object, e.g., a planetary atmosphere
- reflection spectra
the (stellar) light reflected by the object
- emission spectrum
- if the planet is transiting a star, a transit spectrum can be obtained
↗ emission, transmission, and reflection (Gao et al. 2021)
→ planet size at different wavelengths. The atmosphere opacity and therefore its size depends on wavelength.

transmission spectrum

end of module 6
—congrats—